Case Studies
FAR BIOTECH’s VALIDATION
See below how we have achieved validation in several areas with both academic and pharmaceutical organizations.
- STAT-3 PROGRAM
- BLOOD- AND LIVER-STAGE MALARIA
- AFRICAN SLEEPING SICKNESS
- ALZHEIMER’S DISEASE TAUOPATHY PROJECT
- NRF2 ACTIVATOR
- BCR-ABL1
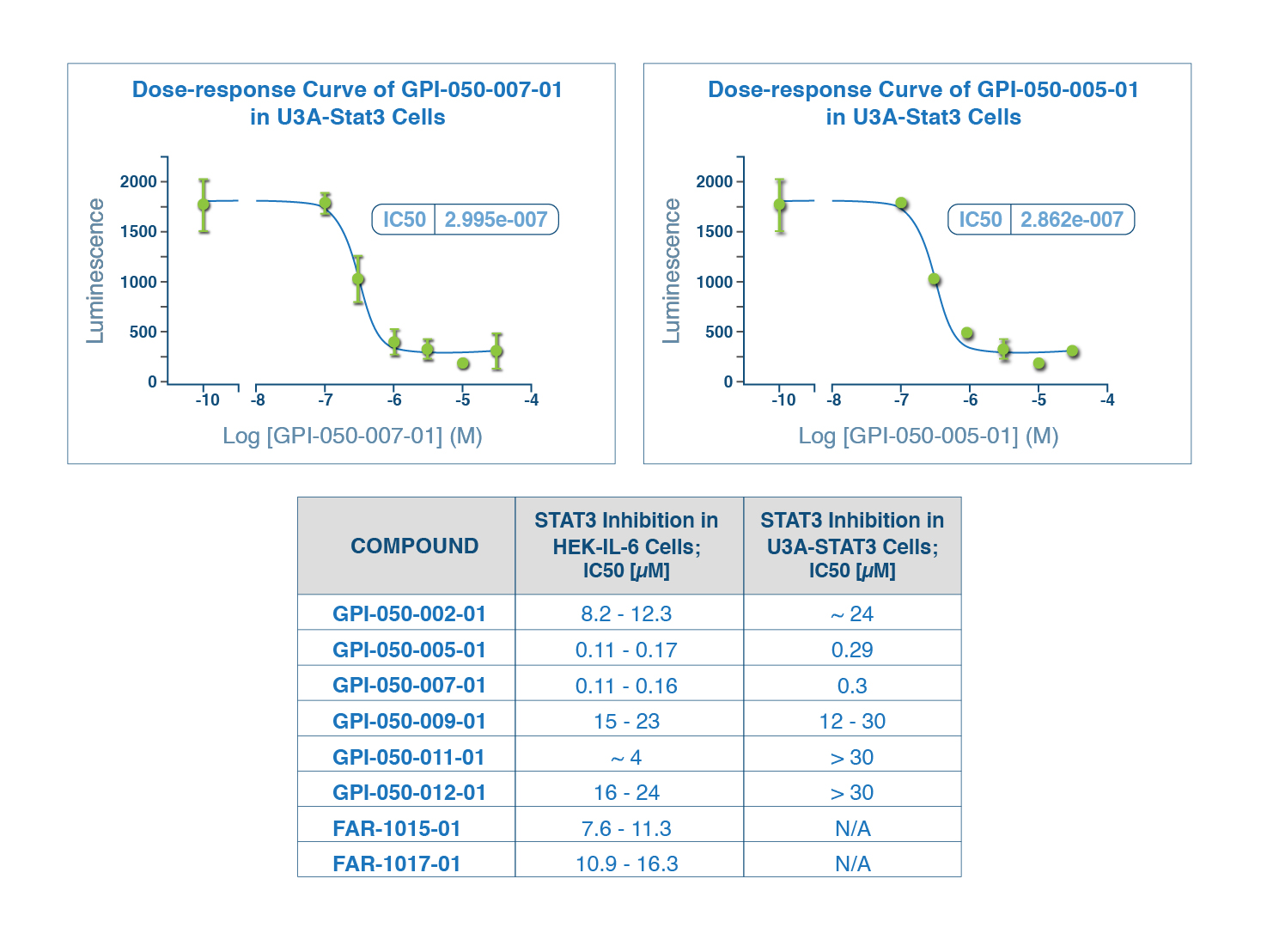
STAT-3 PROGRAM
- Quantum modeling of public compound database identified 12 hits
- Compounds 050-005 and 050-007 exhibited bioactivity at nanomolar concentrations (110-300 nM) in living cells, which is an order of magnitude better than drug currently in the clinic
- Quantum modeling of NCEs yielded several compounds bioactive at low micromolar concentrations
- Currently advancing this program with experimental collaborator
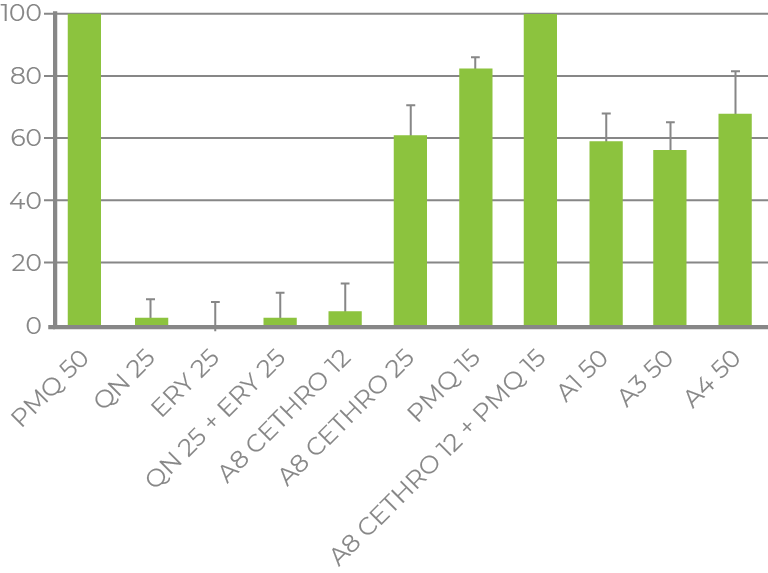
BLOOD- AND LIVER-STAGE MALARIA
- Phase I sponsored by Gates Foundation (Grand Challenges in Global Health initiative)
- Phase II is supported by NIH/NCATS
- Collaboration with Johns Hopkins University
- Discovery of anti-malarial repurposing candidate and NCEs
- Anti-malarial, cytotoxicity, aqueous solubility and hepatic metabolism quantum models
- Cethromycin potency discovered
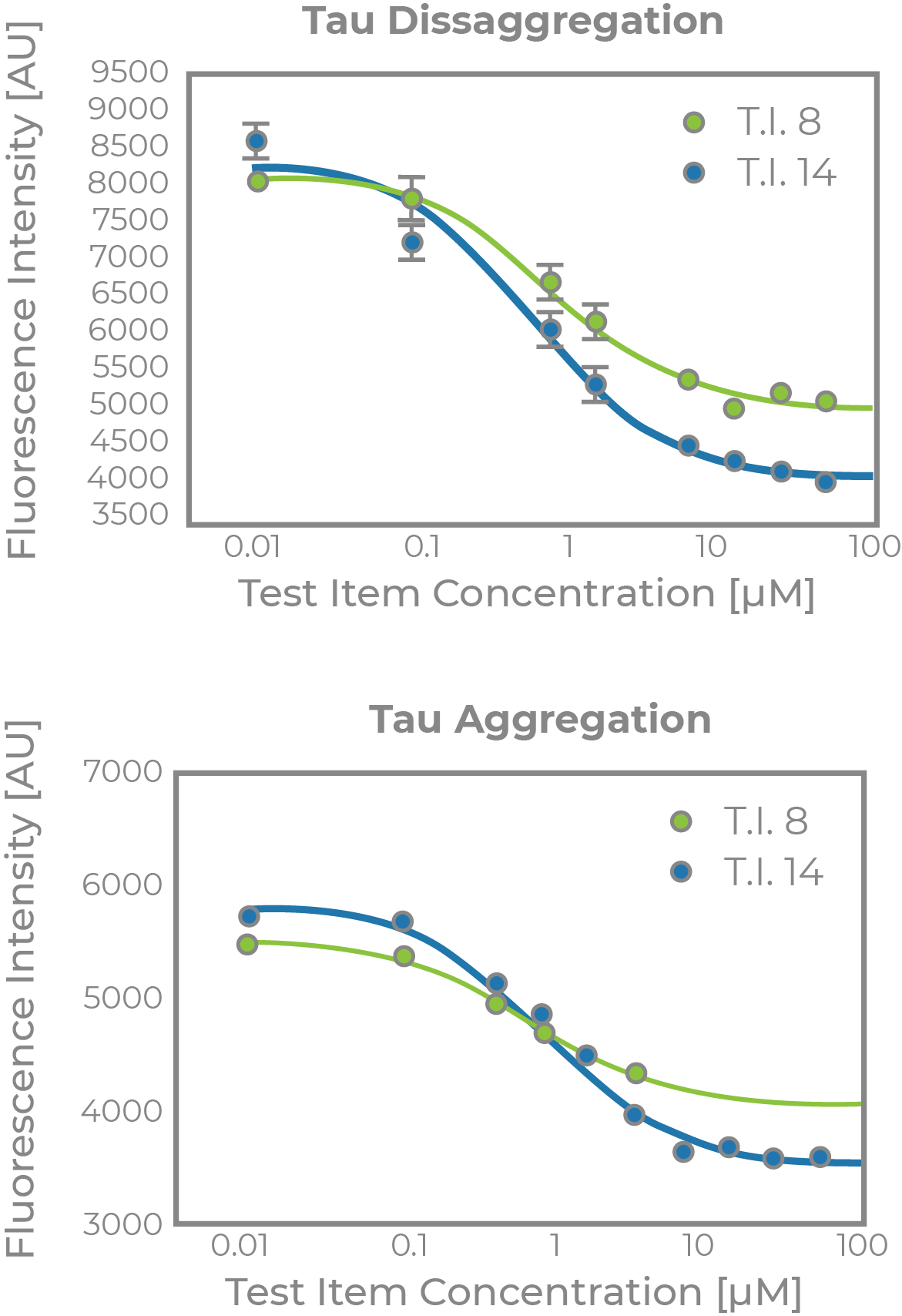
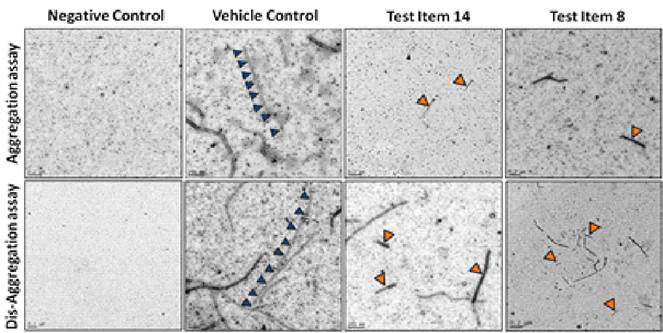
ALZHEIMER’S DISEASE TAUOPATHY PROJECT
- Supported by a Phase I SBIR grant award from NIH/NI
- Collaboration with Dr. Kuret at Ohio State University
- Discovery of novel tau-aggregation inhibitors as potential disease modifying therapeutics for AD
- Tau aggregation, cytotoxicity, aqueous solubility and BBB penetration models
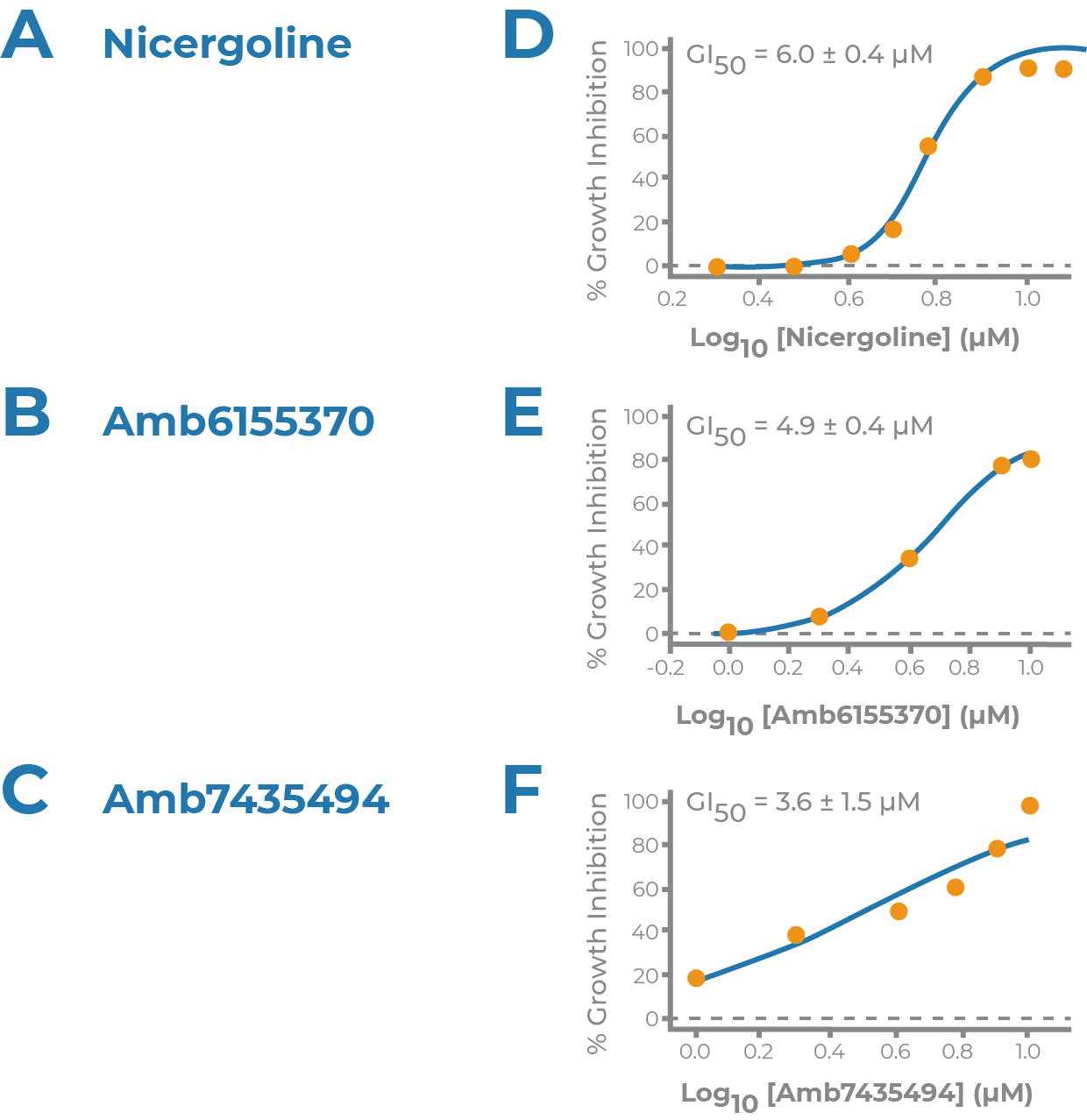
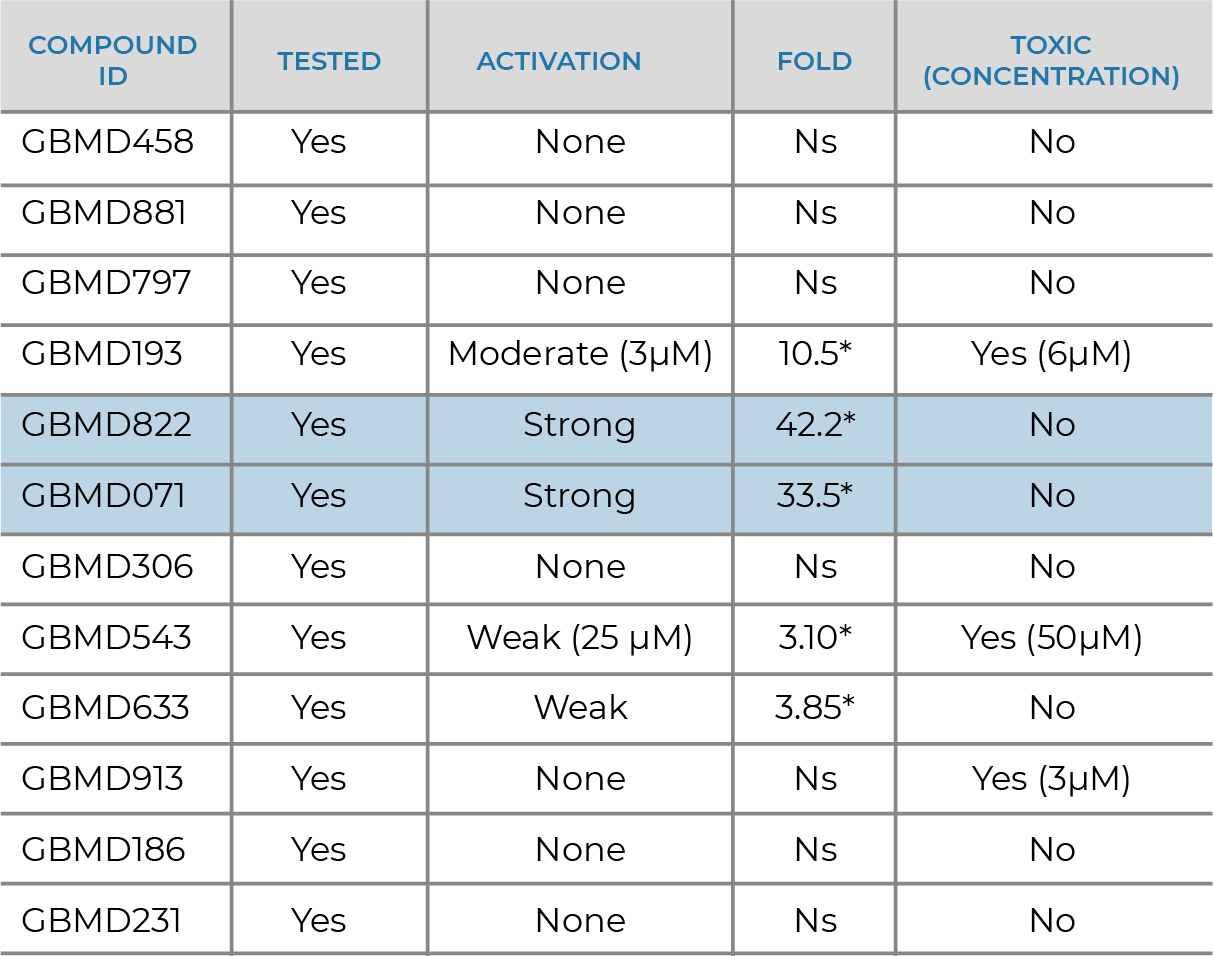
NRF2 ACTIVATOR
- Supported by the Michael J. Fox Foundation for Parkinson’s Research
- Collaboration with Dr. Johnson at University of Wisconsin
- Discovery of novel Nrf2 activators as potential disease modifying therapeutics for Parkinson’s
- Nrf2 activation, cytotoxicity and BBB penetration models
- GBMD822 potency discovered
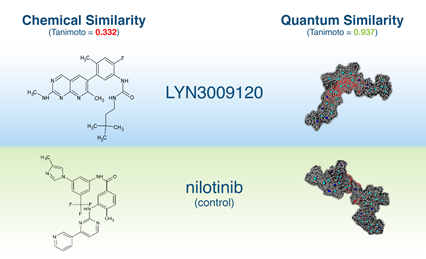
BCR-ABL1
- Collaboration with Huntsman Cancer Institute – University of Utah Health
- Selected 50+ virtual hits, tested against wild-type and mutant BCR-ABL1
- We discovered clinical compound LY3009120, a structurally novel BCR-ABL1 inhibitor when paired with asciminib
- LY3009120 is effective inhibitor of T315I and T315l-containing compound mutants
STAT-3 PROGRAM
- Quantum modeling of public compound database identified 12 hits
- Compounds 050-005 and 050-007 exhibited bioactivity at nanomolar concentrations (110-300 nM) in living cells, which is an order of magnitude better than drug currently in the clinic
- Quantum modeling of NCEs yielded several compounds bioactive at low micromolar concentrations
- Currently advancing this program with experimental collaborator
Blood- and Liver-Stage Malaria
- Phase I sponsored by Gates Foundation (Grand Challenges in Global Health initiative)
- Phase II is supported by NIH/NCATS
- Collaboration with Johns Hopkins University
- Discovery of anti-malarial repurposing candidate and NCEs
- Anti-malarial, cytotoxicity, aqueous solubility and hepatic metabolism quantum models
- Cethromycin potency discovered
AFRICAN SLEEPING SICKNESS
ALZHEIMER’S DISEASE TAUOPATHY PROJECT
NRF2 ACTIVATOR
- Supported by the Michael J. Fox Foundation for Parkinson’s Research
- Collaboration with Dr. Johnson at University of Wisconsin
- Discovery of novel Nrf2 activators as potential disease modifying therapeutics for Parkinson’s
- Nrf2 activation, cytotoxicity and BBB penetration models
- GBMD822 potency discovered
BCR-ABL1
- Collaboration with Huntsman Cancer Institute – University of Utah Health
- Selected 50+ virtual hits, tested against wild-type and mutant BCR-ABL1
- We discovered clinical compound LY3009120, a structurally novel BCR-ABL1 inhibitor when paired with asciminib
- LY3009120 is effective inhibitor of T315I and T315l-containing compound mutants